Geochemistry: Simple Conditions Of Calcite Precipitation
Why is it important to understand the conditions that lead to the precipitation and dissolution of the mineral calcium carbonate (calcite - CaCO3)?
In this note, I provide a summary of some simple geochemical conditions that lead to release of the element calcium from common rock-forming minerals. Most of these conditions occur during surface and near surface weathering of ccalcium-bearing rocks. The release of the element calcium into the environment is the first step leading to the precipitation of the mineral calcite. Some of the basic chemical formulae I use are described in most basic chemistry text books. If interested, you may want to check out the following resources, which focus on chemical weathering:
a) https://opentextbc.ca/geology/chapter/5-2-chemical-weathering/ ;
b) https://www.saskoer.ca/physicalgeology/chapter/8-2-chemical-weathering-2/ and
c) https://books.lib.uoguelph.ca/canadiannaturalhazardsclimatechange/chapter/5-2-chemical-weathering/
The mineral calcite is widespread and is made of calcium carbonate (CaCO3). Several geochemical systems on Earth, including the global carbon dioxide (CO2) cycle (remember Climate Change?), are controlled to a large extent by the dissolution and precipitation of calcite. Therefore, understanding the chemical processes that cause calcite to precipitate, or dissolve, help us understand climate change and the origin of some calcareous habitats, like: a) hot spring tufa deposits (Photo 1); b) marl lakes (Photo 2); c) limy muds that form limestone rock, d) calcite-rich alkali flats; and e) calcite-rich scale (limescale) on rock surfaces.
Photo 1: This tufa landscape is underlain by whitish yellow- to -grey-coloured material called tufa. Tufa is made largely of the mineral calcite. The calcite was precipitated on surface from warm calcareous groundwater. Calciphile plants grow on this tufa substrate. Photo composed at Warm Bay, Atlin Lake, northern British Columbia, by Andy Fyon, July 17/19.
Photo 2: Dry Lake is a marl lake located southwest of Marlbank, Ontario. The white lake bottom of Dry Lake consists of calcareous mud and shells of gastropods. The odd looking cuts in the lake bottom are the relicts of a past mining history, where the lake bottom sediments were mined to make Portland Cement. The narrow linear white ribbons are old railroad lines. Image source: Google Earth.
Calcareous Habitats
Calcite is the main calcium-bearing mineral that occurs in calcareous habitats. Calcareous habitats are unusual and uncommon. They are home to some unusual flora, birds, and insects. Understanding the geological and geochemical processes that lead to precipitation, or dissolution, of calcite help us understand the chemical steps that create calcareous habitats. That, in turn, helps us understand the location and physical features of some calcareous habitats and the types and distributions of flora that live in this unusual habitat.
The chemistry controlling the formation of calcareous habitats can be very complex. Although my synthesis is very simple, it is a place to start to understand some of the geological, physical, and chemical factors that may have played a role in the formation of some calcareous habitats.
Take-Away Synthesis
In case you don’t want the read about the chemical reactions that describe the stability of calcite (CaCO3), carbon dioxide gas (CO2), and water (H2O), here are the four important “take-aways”:
increasing the pressure of CO2 gas in a system causes calcite to dissolve and go into solution;
decreasing the pressure of CO2 gas in the system causes calcite to precipitate from solution;
increasing the water temperature decreases the amount of CO2 dissolved in water and causes calcite to precipitate;
decreasing the water temperature increases the amount of CO2 dissolved in water and causes calcite to dissolve.
And Now, A Little Chemistry
Chemists describe a chemical reaction using reaction equations. The reaction equation describes what happens when components on one side of the equation react together to form components on the other side of the equation. For example:
A + B => C + D
means components A and B react together to produce products C and D. Chemists define A and B as the reactants and C and D as the products.
Reaction directions:
An important convention used by chemists is the direction that a chemical equation moves. A chemical reaction can move in either direction, depending on the conditions. For example, using the imaginary chemical reaction:
A + B => C + D (forward direction)
the reaction is written in a way with the arrow pointing to the right, “=>”. That means reactants A and B react together to produce products C and D. The reaction proceeds in the “forward reaction direction”, from left to right.
Alternatively, we can write the chemical equation such that it shows the reaction moving in the reverse direction, or from right to left:
A + B <= C + D (reverse direction)
For the reverse direction, the components C and D react together to produce products A and B.
Finally, there is a more general reaction notation that tells us the reaction can go in either direction, depending on the conditions:
A + B <=> C + D (either direction)
The chemical reaction can go “forward” (left to right) or it can go in the “reverse” direction (right to left), depending if, or how, we change the conditions affecting the reaction or if a steady state. Chemists also use the term chemical equilibrium. Chemical equilibrium is the state of a reversible chemical reaction in which there is no net change in the amounts of reactants and products.
Calcite Stability
One way to understand how the mineral calcite (CaCO3 ) behaves at, or near, the surface of the Earth, is to consider how carbon dioxide gas (CO2 ) distributes itself between the atmosphere, or soil gas, and water (H2O). Chemists use the word “partition” to describe the distribution of a component, such as CO2, between air and water.
Carbon dioxide (CO2) dissolved in water (H2O):
Our atmosphere consists mostly of nitrogen gas (N2) and oxygen gas (O2), but it also contains a small amount of carbon dioxide gas (CO2). Let’s apply the reaction notation to describe a relationship between carbon dioxide gas (CO2) and water (H2O). That reaction [1] is written:
CO2 in the air <=> CO2 dissolved in water [1]
When a water raindrop falls through the atmosphere, CO2 gas distributes itself between the water raindrop and the atmosphere. The symbol <=> means CO2 gas moves back and forth between the atmosphere and the raindrop. Said differently, a small amount of CO2 gas from the atmosphere gets dissolved in the water. Simultaneously, a small amount of the dissolved CO2 escapes back into the atmosphere. That CO2 exchange continues back and forth between the air and water until a steady state, or equilibrium, is reached. At equilibrium, the amount of CO2 gas in the water remains the same so long as the temperature and the pressure don’t change. If we change the temperature or the pressure of the atmosphere or the water, the ratio of CO2 gas dissolved in the water will also change.
Influence of carbon dioxide (CO2) pressure:
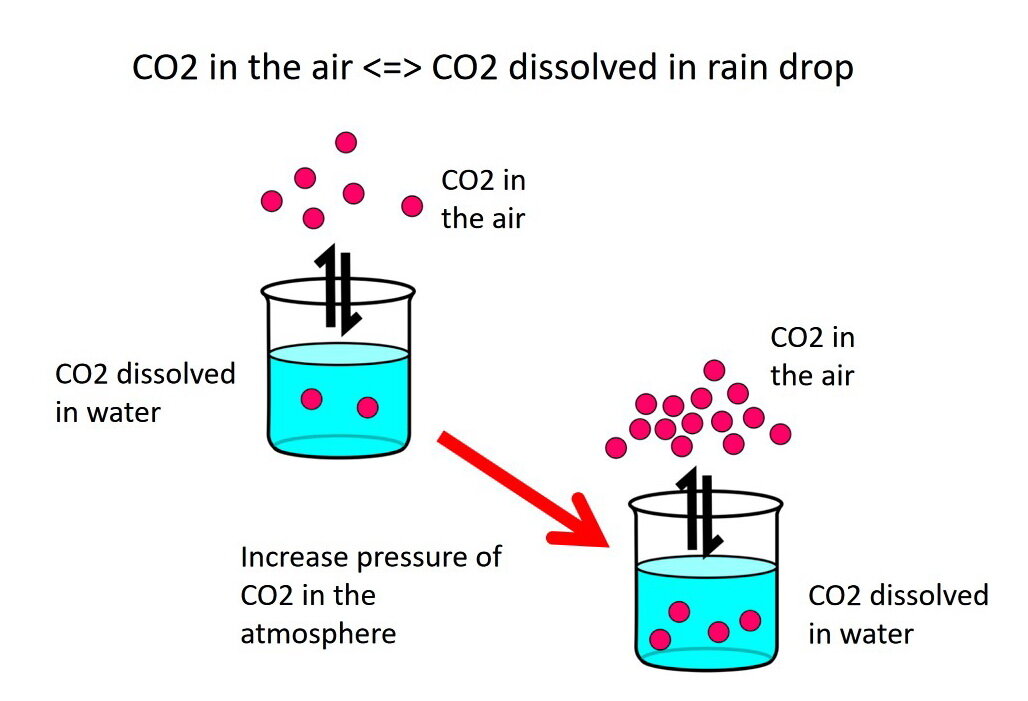
If we increase the pressure of carbon dioxide gas (CO2) in the atmosphere, then reaction [1] adjusts by pushing the reaction "forward", to the right. That is, the amount of carbon dioxide gas (CO2) in the water raindrop, or beaker of water, increases because more carbon dioxide gas (CO2) is pushed into, or is dissolved into, the water (Photo 3). The reaction continues until a new equilibrium state is reached between the amount of carbon dioxide gas (CO2) in the atmosphere and the water raindrop.
Photo 3: Increasing the pressure of carbon dioxide gas (CO2) in the atmosphere, represented by increasing the number of red dots above the beaker, causes the reaction to adjust by pushing "forward" to the right to increase the amount of carbon dioxide gas (CO2) in the water. The reaction continues until a new equilibrium state is reached between the amount of carbon dioxide gas (CO2) in the atmosphere and water. Image by E. Ginn.
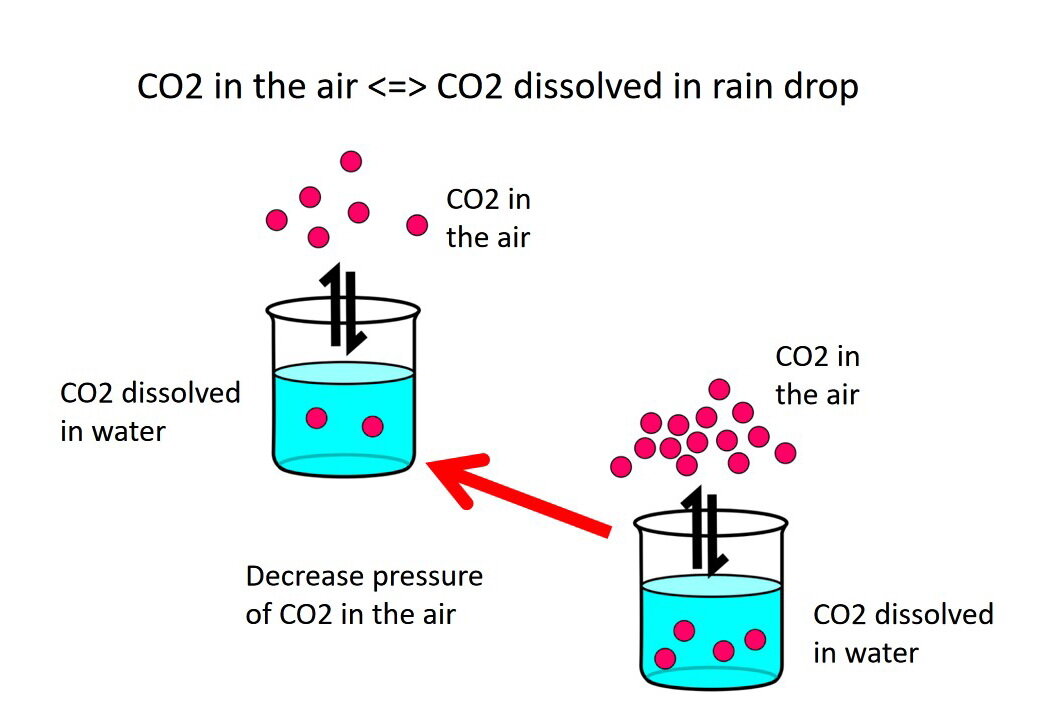
If we reduce the pressure of carbon dioxide gas (CO2) in the air, reaction [1] compensates by pushing the reaction in “reverse”, to the left. That is, the amount of carbon dioxide gas (CO2) dissolved in water raindrop, or beaker of water, decreases as carbon dioxide gas (CO2) leaves the raindrop and partitions into the atmosphere (Photo 4).
Photo 4: Decreasing the pressure of carbon dioxide gas (CO2) in the atmosphere, represented by decreasing the number of red dots above the water beaker, causes the reaction to compensate by pushing the reaction in “reverse”, to the left, to decrease the amount of carbon dioxide gas (CO2) dissolved in the water. The reaction continues until a new equilibrium state is reached between the amount of carbon dioxide gas (CO2) in the atmosphere and water. Image by E. Ginn.
Reaction between the mineral calcite (CaCO3) and carbonic acid (H2CO3):
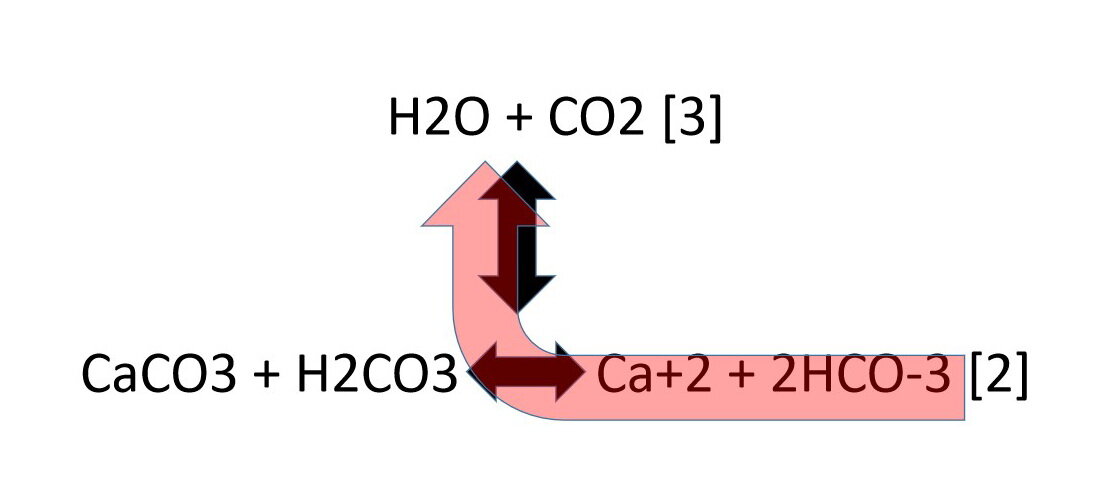
What do these reactions tell us about the stability of the mineral calcite (CaCO3)? One chemical reaction that influences the behaviour of the mineral calcite (CaCO3 ) on, or close to, the land surface is:
CaCO3 + H2CO3 => Ca+2 + 2HCO-3 [2]
Calcite + Carbonic acid => calcium ion + bicarbonate ion
What does this chemical reaction say? Imagine a bowl of water. Add some naturally occurring, weak, carbonic acid (H2CO3) to the water. Then place a piece calcite (CaCO3) in the water. The carbonic acid reacts with, and dissolves, the calcite to create two products: calcium ions (Ca+2 ) and bicarbonate ions (HCO-3), both of which remain dissolved in the water.
How does carbonic acid (H2CO3) form in nature?
Carbonic acid (H2CO3) is made from the reaction between the water (H2O) in the raindrop water, surface water, or groundwater and the carbon dioxide (CO2) in the atmosphere or soil:
H2O + CO2 = H2CO3 [3]
Water + Carbon dioxide gas = Carbonic Acid
Calcite and carbonic acid:
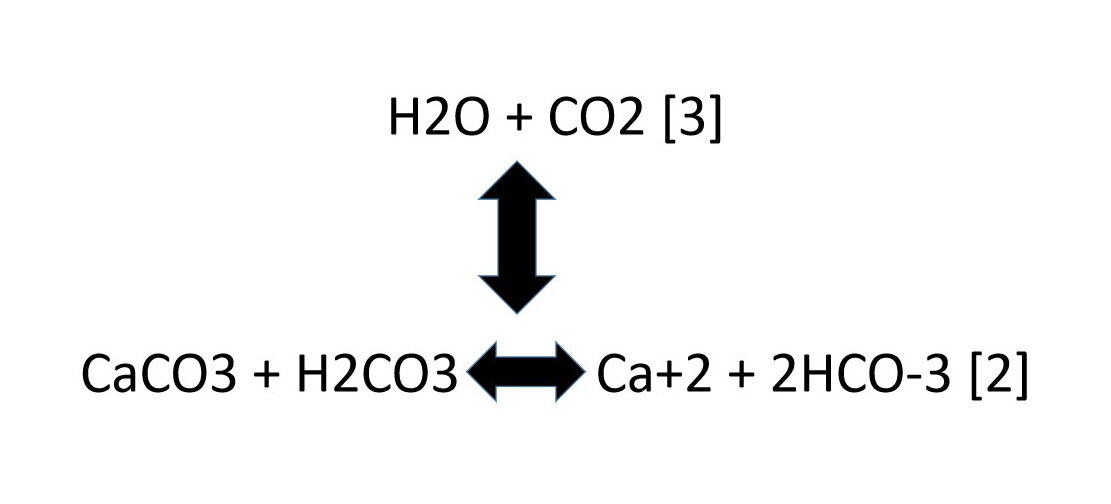
We can write a chemical reaction to show how reactions [2] and [3] relate to each other:
The double-headed arrow symbols show the possible chemical reactions that may take place.
If we mix all of these components together in a sealed jar, at a fixed temperature and pressure, so that no components escape from the jar, the mixture will reach equilibrium, assuming none of the reactants are completely used up. The relative amounts of water (H2O), carbon dioxide (CO2), calcite (CaCO3), carbonic acid (H2CO3), calcium ion (Ca+2), and bicarbonate ion (HCO-3) will be fixed when equilibrium is reached.
Changing carbon dioxide (CO2) pressure:
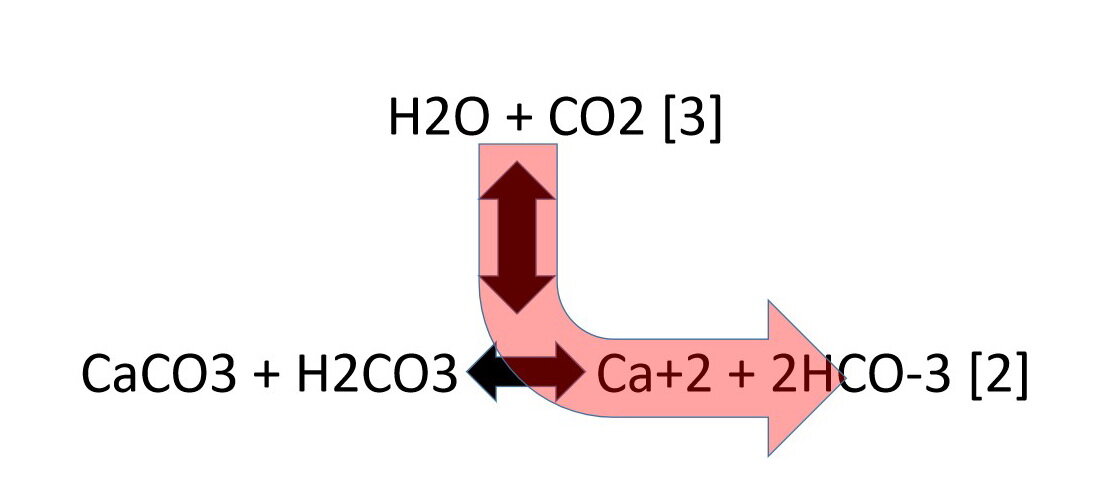
What happens if we increase the pressure, or amount, of CO2 in the jar, while keeping the temperature fixed?:
reaction [3] shifts to the right, or forward, or down in Photo 5, to use up the extra carbon dioxide (CO2) and more carbonic acid (H2CO3) is produced;
increased amount of carbonic acid (H2CO3) pushes reaction [2] “forward”, to the right, and calcite (CaCO3) dissolves (Photo 5).
Photo 5: Increasing the carbon dioxide (CO2) pressure causes calcite (CaCO3) to dissolve.
Said simply, if we increase the pressure, or amount, of CO2 gas in our jar, at constant temperature, more calcite dissolves and goes into solution.
The reverse happens if we reduce the pressure of carbon dioxide (CO2), or pull carbon dioxide “away” from the reaction at constant temperature. In that case, chemical reaction [2] is pulled in the “reverse” direction, to the left, and calcite precipitates (Photo 6).
Photo 6: Reducing the carbon dioxide (CO2) pressure causes calcite (CaCO3) to precipitate.
Changing temperature:
What happens to the amount of carbon dioxide dissolved in the water when we heat or cool the water?
Carbon dioxide gas (CO2) is less soluble in warm water than in cold water. That means, less carbon dioxide gas (CO2) dissolves in hot water compared to cold water. Therefore, if we heat the water, less carbon dioxide (CO2) is dissolved in the water (Photo 7). If we reduce the amount of carbon dioxide (CO2) in the water, equation [3] then shifts to the left (“reverses) to compensate by consuming carbonic acid (H2CO3) to produce more CO2. By consuming the carbonic acid (H2CO3), chemical reaction [2] shifts to the left (“reverses”) to compensate by producing more carbonic acid. Calcite (CaCO3) precipitates (Photo 6):
Photo 7: Less carbon dioxide (CO2) is dissolved in hot water compared to room temperature water. Conversely, more carbon dioxide (CO2) is dissolved in cold ice water compared to room temperature water. Image by: E. Ginn, 2020.
Conversely, if we cool the water, more carbon dioxide (CO2) is dissolved in the water (Photo 7) and calcite (CaCO3) is dissolved (Photo 5).
Restated, if we increase the water temperature, the amount of CO2 that is dissolved in the water decreases and calcite precipitates. If we decrease the water temperature, the amount of CO2 dissolved in the water increases and calcite dissolves.
Practical Applications:
Understanding these simple changes in temperature and pressure can help us understand how tufa (Photo 1), marl lakes (Photo 2), and limescale on a sun-baked rock form and why we don’t see the mineral calcite in deep ocean sediments. Those topics will be addressed in a separate discussion note.
In reality, the chemical conditions affecting calcite mineral stability are complex, but this is a simple analysis to start our understanding.
Summary
The natural conditions under which calcite dissolves or precipitates can be complicated. However, we can simplify those chemical reactions and summarize the influences of pressure and temperature on calcite mineral stability as follows:
increasing carbon dioxide (CO2) gas pressure of a system causes calcite to dissolve and go into solution;
decreasing carbon dioxide (CO2) pressure of a system causes calcite to precipitate from the solution;
increasing water temperature of a system decreases the amount of carbon dioxide (CO2) dissolved in water and causes calcite to precipitate;
decreasing water temperature of a system increases the amount of carbon dioxide (CO2) dissolved in water and causes calcite to dissolve.
Have A Question About This Note?
Andy Fyon: Sept. 27, 2020; Oct. 4,/20.